Bharat Biotech’s Covid-19 vaccine well tolerated with no serious adverse events
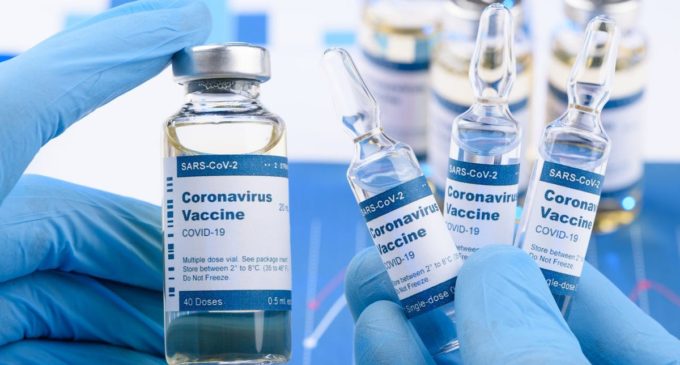
It was a double-blind randomised controlled phase 1 clinical trial to evaluate the safety and immunogenicity of Covaxin (BBV152)
India’s first homegrown vaccine against Covid-19 has shown “robust immune responses” with barely any serious side effects, according to the first scientific study on human trials of the vaccine.
The immunization initiated hearty official and killing neutralizer reactions which were tantamount to those seen in the healing serum gathered from patients who had recuperated from COVID-19, as per the discoveries which have shown up on medRxiv, a preprint worker.
A preprint is an adaptation of a logical original copy posted on a public worker preceding conventional companion audit.
One genuine unfriendly occasion was accounted for, which was discovered to be disconnected to immunization, the discoveries appeared.
It was a twofold visually impaired randomized controlled stage 1 clinical preliminary to assess the wellbeing and immunogenicity of Covaxin (BBV152).
The report specifies that BBV152 is put away between 2 degrees Celsius and 8 degrees Celsius, which is viable with all public vaccination program cold chain prerequisites, and further adequacy preliminaries are in progress.
As indicated by the ‘A Phase 1: Safety and Immunogenicity Trial of an Inactivated SARS-CoV-2 Vaccine BBV152’, after the principal immunization, nearby and foundational unfriendly occasions were transcendently mellow or moderate in seriousness and settled quickly, with no recommended drug.
A comparative pattern was seen after the subsequent shot was controlled. Agony at the infusion site was the most well-known nearby unfavorable occasion.
‘One genuine antagonistic occasion was accounted for. The member was inoculated on July 30. After five days, the member detailed indications of COVID-19 and was discovered to be positive for SARS-CoV-2,’ as per the discoveries.
‘The indications were gentle in nature, yet the patient was admitted to the clinic on August 15. The member was released on August 22 after a negative nucleic corrosive outcome. The occasion was not causally connected with the antibody,’ the discoveries appeared.
To guarantee generalisability, the preliminary was directed on volunteers from assorted geographic areas and financial conditions, selecting 375 members across 11 clinics.
‘In spite of the way that enrolment happened during a public lockdown, which prompted a few operational difficulties, the general member consistency standard was 97 percent,’ the discoveries appeared.
The example size was purposefully huge to empower the induction of important ends with respect to immunogenicity and security, the record said.
BBV152 instigated hearty authoritative and killing neutralizer reactions that were like those incited by other SARS-CoV-2 inactivated immunization applicants, it said.
Two dosages of the immunization were regulated at a volume of 0.5 mL/portion intramuscularly on days zero and 14. The subsequent visits were booked on days 7, 28, 42, 104, and 194. PTI PLB RHL





There are no comments at the moment, do you want to add one?
Write a comment