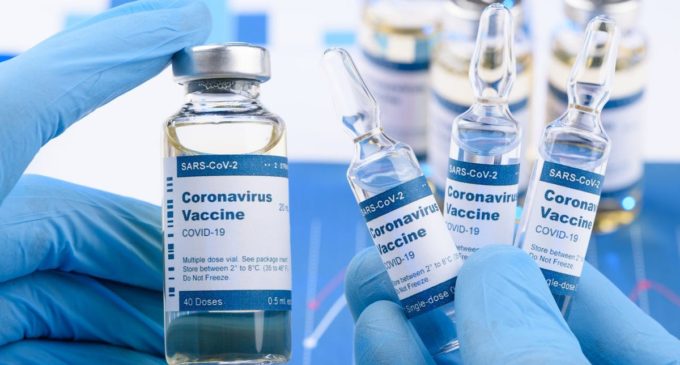
India to have Covid vaccine by June: Kiran Mazumdar-Shaw
Biocon on Friday reported a 23.01% fall in its consolidated net profit to ₹195.4 crore for the second quarter ended September 30, mainly on account of higher expenses.
The buzz around an imminent covid-19 vaccine has raised hopes of a way back to normalcy for the billions affected by the pandemic around the world, said Kiran Mazumdar-Shaw, chairperson and managing director of Bengaluru-based Biocon Ltd. Mazumdar-Shaw is hopeful that the vaccine will be in India by June, but added delivering the vaccine to India’s over 1.2 billion population has its own challenges.
“I anticipate that by January, a portion of different immunizations could be affirmed like AstraZeneca’s or one of our own Indian antibodies like the one by Bharat Biotech. In the event that we finish the clinical preliminaries in the following 2-3 months, even those might be affirmed by January-February. So I would expect that in Q1FY22 we ought to have antibodies accessible in India and different pieces of the world,” said Kiran Mazumdar-Shaw.
Mazumdar-Shaw included that these will be crisis use approval (EUA) simply because one needs to see solidness of reactions before getting the full endorsement.
Biotechnology major Biocon on Friday announced a 23.01% fall in its combined net benefit to ₹195.4 crores for the subsequent quarter finished September 30, chiefly because of higher costs.
“We have been affected by a lot higher innovative work (R&D) spend that has gone up by nearly ₹44 crores and a total deficit of ₹18 crores in forex contrasted with a net addition a year ago. We’ve had different costs and higher staff costs. So I believe that is likewise brought about quieted productivity,” said Kiran Mazumdar-Shaw.
The R&D cost of the organization for the quarter finished September 30, 2020, was at ₹148 crores as against ₹104 crores in a similar time of the past monetary.
The Biotechnology organization had posted a net benefit of ₹253.8 crores for the July-September quarter past financial. Its combined all-out pay remained at ₹1,760.3 crores for the quarter viable. It was ₹1,605.7 crore for a similar period a year back.
Mazumdar-Shaw expects the primary mRNA antibodies will be affirmed before the year’s over. Yet, they won’t be accessible in India since they require a – 80-degree cold chain and that isn’t something which we can deal with here, she said.
In contrast to a polio antibody, Coronavirus immunization will be an intra-strong infusion which can’t be given by ASHA laborers and others. An incredible foundation alongside the following component will be required.
“You will require attendants, specialists, MBBS understudies to convey the immunization. Aside from HR, we have to get a framework for cold chains to be set up appropriately. Likewise, it is an antibody that needs to been allowed twice (one month separated). This gets a ton of intricacy,” said Mazumdar-Shaw.
“You have to follow the strength of the two portions to have information on chain recognizability and accessibility.
I think Aadhaar is the most ideal approach to do it as it permits you to accomplish something at a monstrous scope in such a proficient manner dissimilar to some other nation can do,” Biocon executive included.






There are no comments at the moment, do you want to add one?
Write a comment