Johnson & Johnson pauses Covid vaccine trial as participant becomes ill
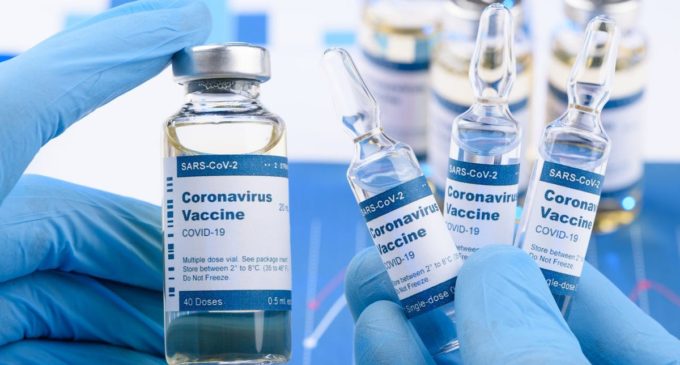
Earlier this month, Johnson & Johnson joined the short list of vaccine makers that have moved an experimental coronavirus shot into late-stage human studies in the US
Johnson & Johnson said Monday it had temporarily halted its Covid-19 vaccine trial because one of its participants had become sick.
“We have briefly delayed further dosing in the entirety of our Covid-19 antibody up-and-comer clinical preliminaries, including the Phase 3 ENSEMBLE preliminary, because of an unexplained disease in an examination member,” the company said in an announcement.
The respite implies the online enlistment framework has been shut for the 60,000-persistent clinical preliminary while the autonomous patient security board of trustees is gathered.
J&J said that serious adverse events (SAEs) are “a normal aspect of any clinical investigation, particularly enormous examinations.” Company rules permitted them to stop an examination to decide whether the SAE was identified with the medication being referred to and whether to continue the study.
The J&J Phase 3 preliminary had begun enlisting members in late September, with the objective of selecting up to 60,000 volunteers across in excess of 200 destinations in the US and around the globe.
Different nations where the preliminaries were occurring are Argentina, Brazil, Chile, Colombia, Mexico, Peru, and South Africa.
This story has been distributed from a wire organization feed without changes to the content. Just the feature has been changed.





There are no comments at the moment, do you want to add one?
Write a comment